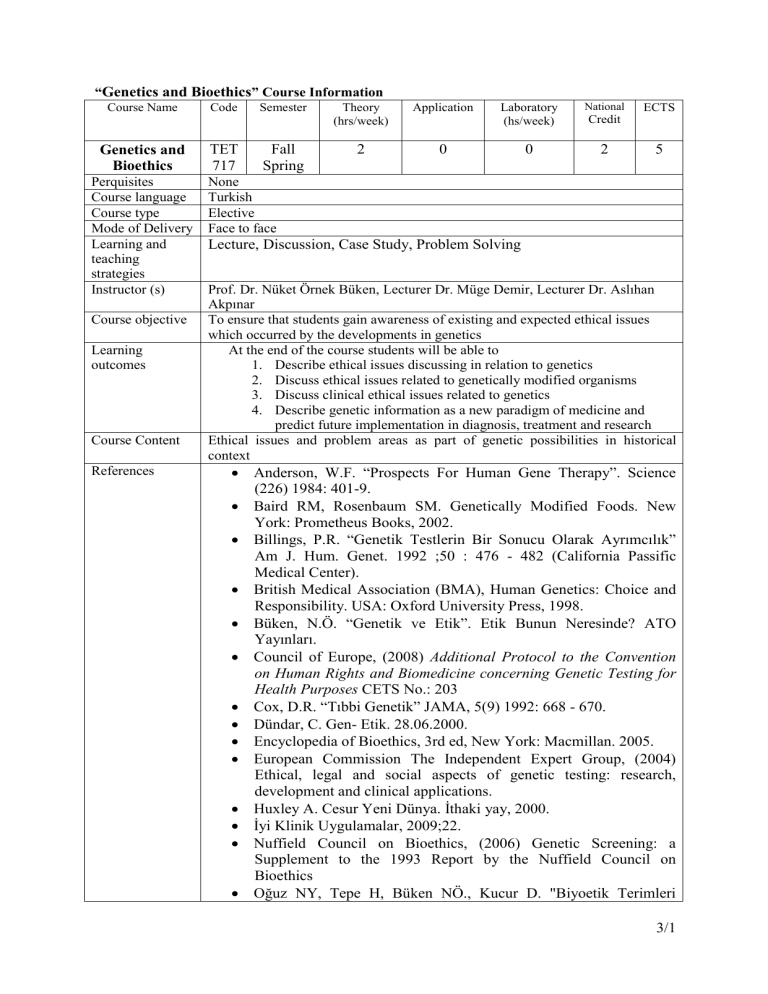
“Genetics and Bioethics” Course Information
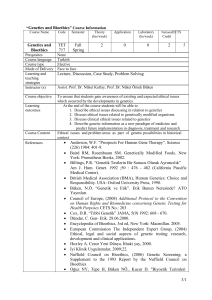
Course Name
Genetics and
Bioethics
Perquisites
Course language
Course type
Mode of Delivery
Learning and
teaching
strategies
Instructor (s)
Course objective
Learning
outcomes
Course Content
References
Code
TET
717
Semester
Fall
Spring
Theory
(hrs/week)
Application
2
0
Laboratory
(hs/week)
National
0
2
ECTS
Credit
5
None
Turkish
Elective
Face to face
Lecture, Discussion, Case Study, Problem Solving
Prof. Dr. Nüket Örnek Büken, Lecturer Dr. Müge Demir, Lecturer Dr. Aslıhan
Akpınar
To ensure that students gain awareness of existing and expected ethical issues
which occurred by the developments in genetics
At the end of the course students will be able to
1. Describe ethical issues discussing in relation to genetics
2. Discuss ethical issues related to genetically modified organisms
3. Discuss clinical ethical issues related to genetics
4. Describe genetic information as a new paradigm of medicine and
predict future implementation in diagnosis, treatment and research
Ethical issues and problem areas as part of genetic possibilities in historical
context
Anderson, W.F. “Prospects For Human Gene Therapy”. Science
(226) 1984: 401-9.
Baird RM, Rosenbaum SM. Genetically Modified Foods. New
York: Prometheus Books, 2002.
Billings, P.R. “Genetik Testlerin Bir Sonucu Olarak Ayrımcılık”
Am J. Hum. Genet. 1992 ;50 : 476 - 482 (California Passific
Medical Center).
British Medical Association (BMA), Human Genetics: Choice and
Responsibility. USA: Oxford University Press, 1998.
Büken, N.Ö. “Genetik ve Etik”. Etik Bunun Neresinde? ATO
Yayınları.
Council of Europe, (2008) Additional Protocol to the Convention
on Human Rights and Biomedicine concerning Genetic Testing for
Health Purposes CETS No.: 203
Cox, D.R. “Tıbbi Genetik” JAMA, 5(9) 1992: 668 - 670.
Dündar, C. Gen- Etik. 28.06.2000.
Encyclopedia of Bioethics, 3rd ed, New York: Macmillan. 2005.
European Commission The Independent Expert Group, (2004)
Ethical, legal and social aspects of genetic testing: research,
development and clinical applications.
Huxley A. Cesur Yeni Dünya. İthaki yay, 2000.
İyi Klinik Uygulamalar, 2009;22.
Nuffield Council on Bioethics, (2006) Genetic Screening: a
Supplement to the 1993 Report by the Nuffield Council on
Bioethics
Oğuz NY, Tepe H, Büken NÖ., Kucur D. "Biyoetik Terimleri
3/1
Sözlüğü", TFK (Türkiye Felsefe Kurumu) Yayını, Ankara, 2005.
Rothstein MA. Genetic Secrets: Protecting Privacy and
Confidentiality in the Genetic Era, New Haven, CT: Yale
University Pres, 1997
Steinbock B, Arras JD, London AJ. Ethical Issues in Modern
Medicine. New York: McGrawHill, 2009.
UNESCO (1997). İnsan Genomu ve İnsan Hakları Evrensel
Bildirgesi, kuramdan uygulamaya.
UNESCO (2003) İnsan Genetik Verileri Uluslararası Bildirgesi
“Genetics and Bioethics” Course Outline Weekly
Weeks
Topics
1.
Basic concepts and history
2.
Introduce of genetic technology to biomedical area
3.
Transgenic foods (Monsanto example) and ethics discussions
4.
Transgenic animal production and ethics discussions
5.
Biopolicy, biosafety, biodiversity, bioterrorism
6.
Human Genome Project (HUGO) and ELSI
7.
Genetic screening and diagnosis tests, PGD, prenatal tests
8.
Gene therapy
9.
Designed babies, the right to be born healthy or modern eugenics
10.
Using genetic information in clinics and genetic counseling
11.
Privacy of genetic information and sharing, DNA banks
12.
Cloning, stem cell researches
13.
Related ethical and legal regulations
14.
A projection of genetically modified future
15.
General preparation
16.
Final exam
“Genetics and Bioethics” Assessment methods
Course Activities
Attendance
Laboratory
Application
Field activities
Specific practical training
Assignments
Presentation
Project
Seminar
Midterms
Final exam
Total
Percentage of semester activities contributing grade success
Percentage of final exam contributing grade success
Total
Number
Percentage
14
2
2
1
10
20
20
50
100
50
50
100
3/2
“Genetics and Bioethics” Workloads and ECTS Calculation
Activities
Number
Course Duration (x14)
Laboratory
Application
Specific practical training
Field activities
Study Hours Out of Class (Preliminary work,
reinforcement, ect)
Presentation / Seminar Preparation
Project
Homework assignment
Midterms ( Study duration )
Final Exam (Study duration)
Total Workload
Duration
(hour)
Total Work
Load
14
0
0
0
0
14
2
0
0
0
0
2
28
0
0
0
0
28
2
0
2
0
1
16
0
16
0
30
32
0
32
0
30
150
Matrix of the “Genetics and Bioethics” Course Learning Outcomes Versus Program Outcomes
Contrubition
level*
Program Outcomes
1 2 3 4 5
1. Highly knowledgeable of ethical / value problems that will be aroused by cutting-edge
X
technology in biomedicine
2. Approaches to value problems will/be aroused in bioethics, health-care ethics- medical ethics
and clinical ethics with environmental and civic awareness; is aware of ethical dilemmas and
describe ethical problem solving methods particular to these dilemmas; develops and applies
original ethical problem solving methods
3. In his/her institution, recognizes ethics committee (research, clinical, animal experiment,
academic...) need and be a leader of founding ethics committees.
4. In his/her institution, gives ethics consultation in any problem about bioethics and biomedicine
to anyone who needs
5. Systematically evaluates, uses and analyzes the institutional and national policies and national
and international ethical and legal regulations about bioethics and biomedical ethics
6. Researches and writes multidisciplinary, interdisciplinary or transdisciplinary, qualitative or
quantitative, national or international projects on current/anticipated issues of bioethics
(medical ethics)
7. Uses current developments in bioethics for the benefit of society considering national values
and conditions with gender awareness; actively participated in establishing policies, guidelines,
national and international ethical and legal regulations about bioethics and bioemedical ethics
8. Be an active member and leader in the national (TTB Etik Komisyonu, TEDMER…) and
international (UNESCO, ICH-GCP…) ethics committees and commissions
9. Prepares and conducts training programmes on bioethics, health-care ethics, medical ethics,
clinical ethics and history of medicine for all level of education - baccalaureate, master’s,
doctorate and when necessary for public -.
10. Evaluates history of medicine with an evolutionary approach and as a part of the history of
science; describes historical development, basic ideas, philosophy and value system of
medicine and profession.
11. Differentiates ground/context and figure in assessing historical phenomenon/events; recognizes
casual relationships and uses history to foresee future
12. Researches and writes multidisciplinary, interdisciplinary or transdisciplinary, national or
international projects on history of medicine using methodology of history.
13. Presents his/her academic knowledge effectively and systematically to the scholarly audiences
oral or written format
X
X
X
X
X
X
X
X
X
X
*1 Lowest, 2 Low, 3 Average, 4 High, 5 Highest
3/3